The Beauty of the Periodic Table
All that we see in the universe in its vastness and complexity is due to chemistry. The subject that often becomes the nightmare of students during school and torments many through advanced university courses, is, along with its sister sciences – physics and mathematics – the trifecta required for us to understand the complexities that surround us and make sense of reality. From photosynthesis by which plants convert the sun’s rays into energy, to energy in the human cell, to transmission of genetic code from generation to generation, to human capabilities to see, smell, hear, and touch, everything is supported by principles of chemistry. Any element that exists in the universe consists of atoms, which can be considered for understanding purposes as the basic building blocks. The atoms are made of positively charged protons and neutral neutrons. This forms the nucleus of the atom. The nucleus is surrounded by orbiting electrons at different levels or shells (very similar to the solar system, with the sun acting as the nucleus and planets in various orbits). The number of protons in an atom is called the Atomic Number, while the sum of the number of protons and neutrons in the nucleus of an atom is called the Atomic Mass. An element can have a version of itself that has a different number of neutrons. This version is called an Isotope of the element, which will have the same atomic number but a different atomic mass.
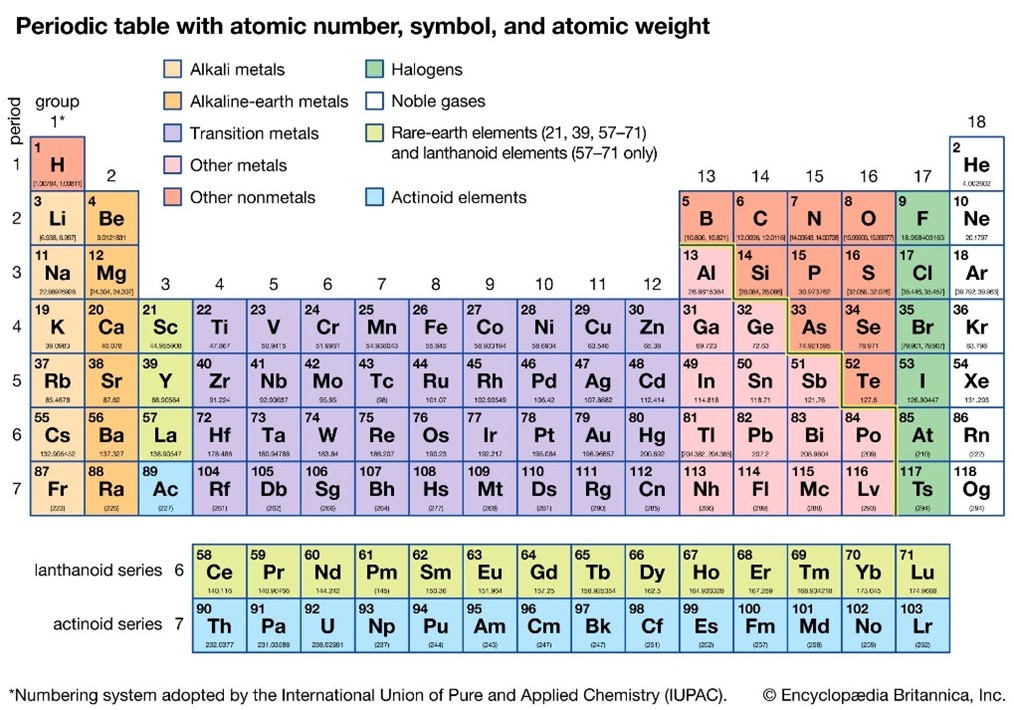
Dmitri Mendeleev was the first to categorize the given elements known in his times into a table. He came upon this structure when he laid out the different elements according to their increasing atomic mass and noticed that elements with a given property regularly occurred in the same relative order. Through this system, he was able to recognize inconsistencies and also prove that certain elements should exist but were still not discovered. It is important to remember that during Mendeleev’s time, the concept of protons, neutrons, or electrons. And that is why his discovery and system of tabulation are considered a landmark. In the modern version of the periodic table, the elements are arranged from left to right in increasing order of atomic number. And each row of the period table denotes the number of orbits electrons occupy around the nucleus of the said element. However, the number of electrons in the outermost orbit will be equal to the number of the column. This helps in understanding the basic properties of the element and also their propensity to react with other elements. Thus, starting from the left and going roughly 3/4th towards the right, the periodic table consists of metals. The overarching definitions of the metals, they will conduct electricity and will be metallic and shiny in appearance. Moving further right, the periodic table is occupied by non-metals and the extreme right column is populated with inert noble gasses.
For example, the left-most column consists of Alkali Metal, which consists of just 1 electron in their outermost orbit. These can lose readily to become positively charged Cations with a charge of +1. These Alkali metals react violently with water to produce hydrogen gas. Similarly, the elements in the second column can lose 2 electrons to become +2 charged Cations. Now, the elements in the second last column on the right – called Halogens – have 7 electrons in their outermost orbit, and they can easily accept an electron to become Anions that are negatively charged. With this information, it becomes easy to understand how these elements will react to each other. For example, a +1 charged Cation will react with a -1 charge Anion to form a compound with an overall charge of 0. Now, if an element in column 1 reacts with a Cation of charge -2, then it would need two Anions to bring the overall charge to 0. And thus, with the categorization of the elements on the periodic table, order is brought into the complex world of chemistry.